posted by Dave Arnold
As I mentioned in my last post: Last week I participated in a seminar at Tales of the Cocktail entitled The Science of Shaking. The panel was put together by moderator Eben Klemm (head of bar programs for the B. R. Guest restaurant empire, well-known innovative cocktail guy, and former biologist) with Alex Day (famed bartender from Death & Company and Franklin Bar) and myself as panelists. Yesterday, we went over some basic cocktail science. Today we are talking simple results.

Dave, Eben, and a lot of Montezuma. Alex Day photographing.
Question
When it comes to determining the final temperature and dilution of a shaken drink, does the type of ice in the shaker or the shaking style really matter?
Short Answer
No (within limits).
Important Note
We did not address drink texture in any way. We first wanted to understand temperature and dilution. Texture is vitally important. We hope to address that in the future.
Assumptions
- Bar ice is 0° Celsius (it isn’t stored in a freezer). This is important. If your ice is in the freezer, it can chill your drink before it starts to melt. The ice will lower the temperature of your drink while getting warmer till it hits 0° C. After the ice gets to 0° C, it doesn’t get any warmer. ALL FURTHER CHILLING IS DONE BY MELTING ALONE. In a bar situation, all chilling is done by melting. There is no chilling without dilution.
- You use “enough” ice. We did initial experiments that showed that using too little ice results in poor chilling and greater dilution. The benefit of adding more ice plateaus at a certain point so that it neither helps nor hurts the temperature or dilution. I don’t have exact numbers for the plateau point (I lost my old data cause I’m a jerk), but using Kold-Draft ice, Eben and Alex shook a 100 ml gimlet with one cube, two cubes, three cubes, and up. They were able to keep getting better results up to at least 5 cubes.
- You don’t use ice so broken down that it carries a huge amount of water with it. The volume of an ice cube goes up as the third power of its size, but the surface area goes up as the square of the size. Small ice cubes, therefore, have a larger surface area per gram than large ice cubes. Since water resides at the surface of wet ice, immense amounts of surface area will unfairly add to dilution.
- You shake “enough.” Putting ice in the drink and walking away doesn’t constitute shaking. You need enough agitation to get fresh drink in contact with the ice. It doesn’t take a whole hell of a lot, as we shall see.
What We Did
- I made some temperature recording shakers by soldering together some type K thermocouple wire, lightly coating the end with heat conductive epoxy (to prevent wicking into the thermocouple insulation), drilling holes into some cocktail shakers, and sealing the thermocouples inside with some steel-reinforced epoxy. These were wired into a Measurement Computing 8 channel thermocouple receiver attached to my computer.
- We took a whole bunch of Montezuma Tequila and Calypso white rum and verified their alcohol content with a refractometer calibrated for ABV (alcohol by volume). All the bottles measured 38% ABV instead of the advertised 40%. We then cracked open some liquor that cost more than five bucks a bottle and it also registered 38, so we learned that Montezuma wasn’t ripping us off. Our refractometer was a bit off.
- We put all the liquor in a controlled water bath set at 75° F. This was our “room temperature.”
- We put a bunch of Kold-Draft ice and a bunch of small ice (made by our machine) into a Randell FX adjustable refrigerated drawer set to 0° C and let it equilibrate for 2 hours. The side of a cube of Kold-Draft measures 1.25 inches across. Many bartenders prefer it because the say it dilutes drinks less. Some people also think it is somehow “colder.” Our small cubes measured .75 inches across a side but had a large simple which increases its surface to volume ratio significantly.

Type K thermocouple in the can

Kold-Draft ice vs standard ice
- For every shake we weighed out similar quantities of ice, recorded the weights, and shook with 100 ml of room temp liquor (deviations of weight in ice are presented at the bottom of the post).
- After every shake we strained the drinks into a fresh room temperature Dixie cup and measured its temperature with a calibrated thermocouple probe (the straining involved some snaps of the wrist to ensure we got all the drink out).
- After the temperature was taken, the drink was poured into a labeled plastic bag sealed and thrown into a 75° F water bath. The residual ice was also bagged and thrown into the water bath.
- After the samples reached room temperature, they were weighed and alcohol level was checked with the refractometer.

Bags O Cocktail

The full test rig
Notes: Yes, we corrected for the differences between weight and volume, and alcohol by volume and alcohol by weight in our calculations. No, there was no sugar or anything else in the liquor to throw off the refractomer readings.
First Experiment
Does type of ice or shaking style affect how cold a drink is or how fast it gets cold? No.
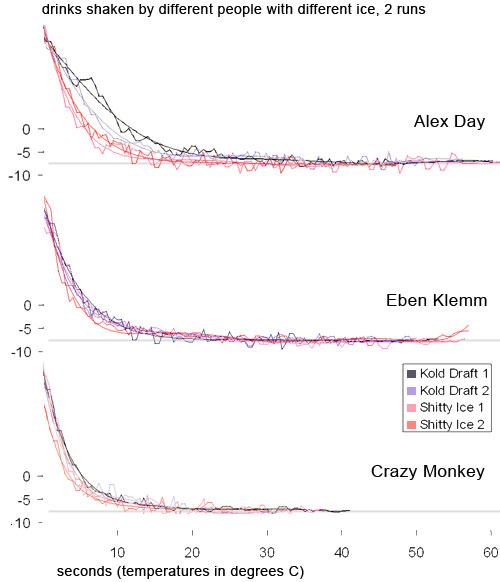
The shaky lines are the actual bits of data from the thermocouple. They go up and down as the drink was shaken. The smooth lines are Excel’s 6th order polynomial curve-fit to the thermocouple data (whoa). Crazy Monkey is me, so named because I shook as hard as I could. I shook so hard that by the end of the shaking I couldn’t move my arms and had to jump up and down to keep going. Notice that even going crazy monkey, all of our final temperatures are about the same, regardless of shaking style and regardless of type of ice. Also notice that the smaller ice is marginally faster than the Kold-Draft, but not by much (that makes sense because it has more surface area). In fact, with the exception of Alex’s first shake (which was the first shake of the day so it might be an anomaly), all the shakes had almost leveled out by 12 seconds. After that we only gained a degree or two of cooling. A degree ain’t no big deal. So much for big ice being “colder.” If anything, the reverse is true. So much for needing to shake really hard. You just need to shake “hard enough.” We don’t know what the minimum “hard enough” is, but we know that a normal bartender’s shake is hard enough. Speaking of shaking hard…
Second Experiment
Does shaking significantly raise the temperature of the drink due to friction? No.
We made “ice cubes” that were exactly the size of Kold-Draft cubes out of a 3% low acyl gellan/water gel. 3% gellan is pretty hard, but the cubes should act like ice cubes in terms of friction. We brought the warm-draft cubes up to room temperature in our water bath and shook as usual.

"Warm-Draft" cubes, blocks of 3% low acyl gellan gel, next to Kold-Draft cubes
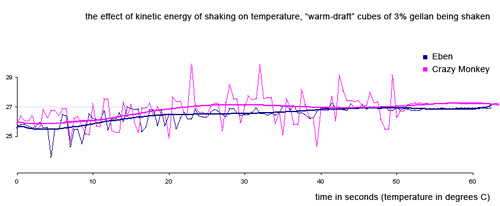
Eben did a normal shake—for 60 full seconds! I went one better and did the crazy monkey shake again (tiring). Note that even with all that shaking, the temperature of our 100 ml drink never rose even a full 2 degrees. That kind of temperature rise would be counteracted by less than 2 grams of ice melting. We found the friction effect insignificant.
Third experiment
Does chilling the shaker and the type of shaker matter? Yes, a little bit.
Alex Day shook some drinks with Kold-Draft cubes using different shaker configurations.
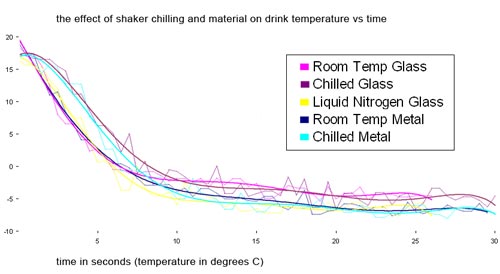
Glass fared worse than metal unless it was chilled with liquid nitrogen (which is cheating). Metal reached a lower final temp than glass did. In fact, room temp metal chilled slower than a chilled glass but got to a lower temperature. Presumably this is because the glass absorbs more heat. I don’t know why the room temperature glass curve is so steep at the outset. Fluke?
Finally, the most important experiment
Does the type of ice affect the final temperature, the final ABV, or the final dilution of the drink? No!!!
Alex Day did all the shaking on this one. He took Kold-Draft ice and shook it with 100 ml of Montezuma for 3, 5, 8, 9.5, 12, 13.5, 20, and 40 seconds, then took crappy ice and shook it with 100 ml of Montezuma for 3, 5, 8, 9.5, 12, 13.5, 20, and 40 seconds. Those numbers were chosen because they were benchmarks for temperatures and times we had achieved in our prior shaking tests. When we analyzed the results, we noticed that the final weights of products we ended up with was lower than the starting weight in every shake. Our first reaction was WTF? Our explanation is that there is a certain “angel’s share” to cocktail making. You lose some while straining, you lose some while shaking, and so on. Here is how much was lost from each sample:
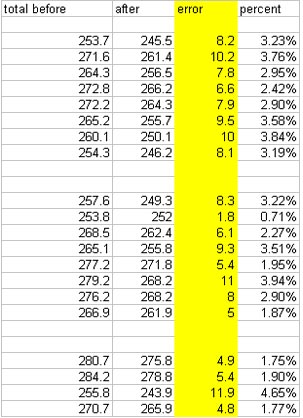
WTF! angel's share for cocktails?
Because of the loss, when determining dilution we used both ABV and the amount of ice we had melted into the drinks as a guide. Here are the results of the tests:
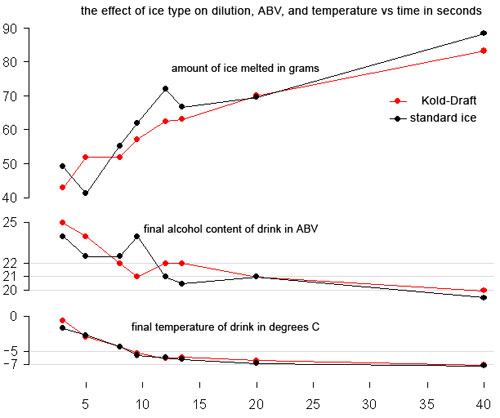
Wow. The curves for standard and Kold-Draft cubes are almost the same! What’s really amazing is that after about 10-12 seconds you get less than 2 degrees extra cooling and between 20 and 40 seconds of constant shaking you only lose 1-1.5% ABV. We thought those results were pretty amazing. Between 20 and 40 seconds, we had only melted 20 grams more of ice. These numbers are in line with strict calculations: 100 ml 40 ABV booze + 70 ml water = 170 ml at 23.5 ABV, about (we got 21), 100 ml 40 ABV booze + 90 ml water = 190 ml at 21 ABV (we got 19.5-20). Remember, however, that our refractometer was reading a bit low.
What does this all mean? In terms of temperature and dilution, your style of shaking and the ice you use probably doesn’t matter. The length of time you shake probably doesn’t matter. All of these are probably vitally important to the texture and look of the drink. Eben put it best when he said that these results should set you free to develop your own style of shaking because you no longer need to worry about time and temperature.
One last thought: We all know that small ice dilutes more than big ice in the glass (or in the shaker if you let it sit there long enough. How do we reconcile that knowledge with the above data? Simple. As the drink sits, it absorbs heat from the surroundings, melting the ice. In a non-agitated situation (like sitting on the bar), big pieces of ice, with its large volume and low surface, will melt slower and dilute your drink less.
deviations in ice weight for different shakes/different ice graph
deviations in ice weight for dilution/temp/ice graph

I’m wondering if the refractometer “error” is due to something dissolved in the booze. The error is inthe right direction and it might not take much.
We wondered that also. We checked a new bottle of Absolut vodka, which also registered 38, Tanqueray which registered low (I forget the number cause Tanq isn’t 40 ABV), and another “premium vodka,” I forget which one, that registered 38 ABV. I tried to adjust the reading up to 40 but then my distilled water read as 2 ABV.
With regards to the small percentage of losses, another possible reason could be the change of density of the drink.
Mass = Density x Volume
And Density changes with increasing/decreasing pressure and/or temperature.
So, if the density of your alcohol reduces due to a decrease in temperature, you will get a weight “loss” of your drink!
Howdy HY,
We correected for weight. The density of the original booze was .94 g/ml at 75F (I think, I’d have to go look it up). Every other calculation was done by weight, so density shouldn’t have been an issue. We actually lost grams.
Moment of inertia is playing a good role in this, Isn’t it?
Hi Science,
I don’t follow. What do you mean?
Regarding the angels share, the properties of a liquid and a surface that make the rotovap so effective are in play with a cocktail shaker and a glass.
You can’t shake off those pesky laws of physics.
Working out the (I’ll follow up with numbers) the 4ml-7ml makes sense for losses with adhesion, given two standard (Libby pint glasses as references Top diameter 3.50″, bottom diameter 2.375″), however the 15ml losses are interesting and cannot be explained by adhesion.
I know it. It’s weird. It might be spray from the initial shaking. I have to try and run the experiment again.
Dave:
The Alcohol Density you mentioned above is correct.
-Getting OT’d
Wait! What if I want my drink stirred, not shaken? Could you repeat the experiments with a stirred drink (I know there is a strong contingent out there in favor of stirred martinis rather than shaken, James Bond notwithstanding). Especially if you’re going to repeat for taste and texture.
Howdy Ian,
Eben did want to do something on stirring as opposed to shaking. I probably won’t have time to work on it till he forces me to, so we’ll have to wait and see. My feeling is that, from a chilling perspective, stirring is a less effective form of shaking. I predict higher equilibruim temperatures and greater dilution (if actually taken to equilibrium), but I could very well be wrong.
Would you predict that these results would be scalable down to, say, roughly cracked ice and up to the fist-sized lumps of commercial block ice some places are using to shake with?
I don’t know about craked and fist sized. You’d need a lot of agitation with fist size and you’d have to shake all the water off the cracked ice.
This is great research! Thanks for (1) doing the experiments and (2) writing up / posting your results.
Thanks for posting this! I had missed the session at Tales, and really wanted to see it.
And I agree with Ian, we need a stirring version of this! :-> Relatively “casual” tests that various folks have done appear to show that both do about similar jobs, but I don’t think anybody has done a serious study of this yet.
Howdy Robert,
Well, if Eben and Alex are up for it we can do some stirring. One of the questions I’m really interested in is how to address drink texture. The more I think about it, the harder it is to quantify. Temperature and dilution are realtively easy. At the seminar, a lot of ideas were presented to address texture, such as measuring density, etc. I think as a first test for texture we need to go qualitative, not quantitative. Get a taste panel, several agreed shaking experts, an agreed recipe, and do it blind.
I wrote a chaptor in our International Bartending Level2 book about ice.
Its basicly a chaptor where I give my student the tools to experiment for themselfs.
We test use of ‘regular’ ice (hozizhaki ice cubes wich are similar to cold draft) vs double frozen ice (hozizhaki ice cubes left over night in a -20 degrees celcius freezer (wich more and more bars are doing in europe))
And things like frozen glasses vs room temp glasses/ pre chilled glasses/ frozen stir glasses ect…
But a lot of my student choose to do something with stirring vs shaking. A lot of the time they test drinks like Manhattan’s or Martini’s.
To make a verry long story (because with the help of my student we have a lot of results by now) short;
Shaking a drink will result in a lower temperature a higher dilution and (duh) a lower alcohol by volume as the same drink stirred.
I uploaded our Bartending Level 2 book to http://www.share.windkracht8.com if you want to have a look. Just click on Wouter and you’ll see a pdf called bartendingbook.
P.S. Robert, I wrote a chaptor in there aswell about The History of Alcohol. Sounds familliar?..
I attended your seminar about the toppic on Tales to see if two people on the other side of the lake made more or less the same time line.
And we did actually.
Altough I din’t have that cool fact about distilling with the use of wool.
How cool is that!?
Great piece of work Wouter, just one comment about the history of Angostura in the Manhattan section. The town of Angostura where Siegert formulated the bitters is in Venezuela not Argentina.
So, what I’m coming away with is a 12 second, regular shake with regular bar ice will achieve satisfactory results? Anything beyond that will not affect temperature and dilution of the drink to a noticeable degree?
Yeah, that’s what the data seem to suport.
Hi,
THANKS FOR SHARING THE RESULTS OF YOUR EFFORTS!
There´s somethign that caught my attention
when comparing the charts of ice type, ABV.
A logical thought will tell use that the more ice is melted, the lower the ABV, but if you see at 9.5 secs the dilution with the standard ice doens´t matches with this logical thinking, while the Kold draft does it (meaning that standad ice reads ABV about 24 and melting rising while Kold draft reads 21 ABV and ice melting rising too)
This appears to stabilize (?) and follow logical hinking at 20 secs, ABV lowering and melting riseing in both ctypes of ices following a similar behaviour.
What do you think it is the reaosn for this?
What do you think it goes smoother after 20 secs, than from 0 secs to 20 secs?
Cheers!
LR
That is strange. I don’t uderstand it. My guess is that it is an abberation and if we did more tests the curves would smooth out.
That is an extraordinary piece of work. Highly appreciated.
Great post. Just one question: if the length of shaking-time and the type of ice doesn`t matter why is my Pegu Club shaked with crushed ice for 40 seconds a watery mess? Or isn`t it a watery mess?
Well, I’ll have to do a test with crushed ice. My guess is you probably get most of the water at the beginning as liquid already on your ice. That starts your cocktail in a pre-diluted state, which only leads to more dilution. That is only a guess. I’ll do some tests but, as I said, I bet most of your over-dilution happens early.
Well, Dave, that was a joke. What I meant is if you taste a cocktail, for example a Pegu Club, witch is shaken a long time, for example 40 seconds, with bad wet ice the result is a watery mess. Or isn`t it?
P.S. I don`t understand the grafic of amount of ice melted in grams. Can you give me numbers? For me it seems like a lot of grams that melted.
Thanks Cocktail Guru!
Totall overshight on my part.
Very interesting, thanks for sharing. I am looking forward to hear about the stirring experiments!
best
Elvira
Oliver:
Our data suggests that your 40 second Pegu is watery partially because you shook it for 40 seconds. That’s a long time in cocktail land. In doing our studies above we were pretty stunned at how perceptable in taste minor variations in ABV were, even at such low temperatures.
I, too would like to repeat our experiments with crushed ice. I suspect that crushed ice may lead to increased dilution because you pack more of it into a shaker.
I would like to repeat our work with crushed ice. I suspect
Love ya work, would also love to see similar for stirring…
Thanks for your generosity in sharing this…
RP
I’m curious: Did you do any looking at how shaking style and dilution interact? My sense is that aggressive shaking with enough room in the shaker for the ice to really move around and hammer back and forth results in greater dilution. I often double strain my cocktails through a fine mesh strainer, and have noticed that in a cocktail that starts out with 3 ounces of booze, I can often strain out as much as a tablespoon of fine ice crystals in the fine mesh strainer. I have also found that packing the shaker with more ice and using a less aggressive shake results in lower dilution and far less production of fine ice crystals. Both conditions would be well within the amount of ice you suggest is “enough” — so presumably the main diffrence is in the shaking style.
Also: When you strained the drinks, did you double-strain, or did you simply strain through the Hawthorne? If the latter, did you notice a significant amount of ice crystals in the poured-out samples?
Howdy SlKinsey,
You are, in fact, anticipating our next cocktail post! Stay tuned for subjective shaking tests at Pegu Club with an all-star cast.
Heh. Nice. I was almost down there myself on Monday, but couldn’t get away.
I await the results with keen anticipation. This is interesting work.
Depending on what your results show, I also wonder whether it might make sense to design the shaker a bit differently from the usual “Boston shaker” two-cups design. I have an old 1950s era stainless steel shaker that consists of a very large bottom cup and a more or less flat top with ~3/4 inch sides that fits down inside the rim of the main cup. There is a small cap flat cap and inset strainer near the side on the top piece, but I never actually strain out of this — I just use the cap as a “release valve” so that I can easily lift off the top piece and strain as normal using a Hawthorne. Anyway… a technique I’ve found that works very well with this shaker is to fill it completely with ice, slap on the cap and shake briskly but with a movement that is small in extent. At first the ice doesn’t move at all, and the liquid more or less washes over the ice inside the shaker. Then, as the ice melts a bit, there is a little bit of movement of the ice. The drinks turn out very cold and with very little chipping of the ice and the extra dilution that goes along with ice chipping. Easy to use as well.
Oh… another place you might consider visiting for some experiments is Dutch Kills in Long Island City. All their ice is hewn from gigantic blocks of clear commercial ice. So, when they shake a cocktail, it is with a single, fist-sized lump of this big ice. That more or less extends your earlier work out as far as it can possibly go.
– Sam
Sam:
Anytime you can “overwhelm” the substrate with a magnificant amount of ice, as in your I Like Ike Strainer, you are of course tilting the temperature dropping efficiency in your favor. I have 2 questions:
What kind of drinks do you make in this way?
Doesn’t this seem more like a horizontal stirring?
Hmm. I guess I’m not sure what you mean by horizontal stirring. I do think it’s good to use a massive amount of ice, however.
I’m probably not describing the process very well. It’s not like the ice doesn’t move at all. It’s just that, with the shaker space more or less completely filled with ice at the beginning, it takes a shake or two before the ice starts to move. And when the ice does move, the extent of the movevement is less (because there is less room in the shaker). At the end, the level of the ice has usually gone down to around 2/3 of the way up the shaker — not so much because it’s melted to that extent, but rather because the cubes have been rounded off by the shaking and fit much closer together.
As for what drinks I might shake this way. . . Not ones that need a lot of churning, obviously. So I wouldn’t do an egg white drink this way. But I like it for drinks that I want to be as cold as possible, but would like to tightly control dilution. And, as a general rule of thumb, I don’t like ice chips in the drink. So, for example, I like to do a lean Daiquiri this way.
I also find that it works well for drinks that I would like to shake very hard, because while the liquid is really flying around in the shaker, the ice isn’t moving so violently and there is less shattering of the ice. On the other hand, if I have something like mint in the shaker, where aggressive shaking might have a tendency to overwork the herb and bring out those brackish “bruised” flavors, I can do a fast kind of twisting shake (as opposed to a straight up-and-down shake) that is perhaps only 3 inches in extent, and get good aeration without tearing up the mint.
I should bring my old shaker down to Audrey’s and see what a few of the guys think about it. Mainly, my thinking about the standard “two cups” arrangement is that I’d like to get more ice in there and bring a bigger (negative) thermal load to the game.
I wonder what sort of hypothesis you guys started with? If I look at the figures I do see differences but I am only left to wonder whether they are significant. An initial hypothesis would be helpful here as it could be either rejected or accepted. Although, a third option could be that the experiment didn’t have enough statistical power in order to test the hypothesis. At least some initial theoretical approach would have been nice in order to investigate what sort of differences would have been expected. I am personally not amazed by these figures. As in, they don’t seem to counter theories telling there is a difference between shaking styles or types of ice in temperature and dilution. The short answer ‘no difference’ seems very shortly to me. What numbers did you expect beforehand when making your assumptions?
I also have doubts about the dilution measurement. Since the negative values indicate that not all liquid is measured it seems very clear to me that the determination is invalid. I personally prefer to measure dilution directly, for instance, by using coloured ice in combination with colorimetry. You say you used abv in the end but it doesn’t seem so.
The figure showing the amount of molten ice, abv and temperature has the nasty effect that the graphs don’t match theory in which amount of molten ice decreases abv and temperature. Where is the sink and how trustworthy are the numbers?
Another remark about the zero degree temperature of the ice. This is very difficult to determine. Incubating the cubes at zero degrees for two hours may get the surface at zero degrees but the core of the ice cubes might still be well below zero. I also wonder why the graphs show that the final temperature of the drink drops below zero if the cubes are supposed to be at zero.
The comment at the end is a bit strange. You first determine/claim that in an agitated situation, no difference can be seen between different types of ice. But at the end you make the statement that in the glass the bigger ice will cause less dilution.
My final note: I believe that the small differences might have large influences. This graph with the different shaker material shows differences which seem small but could have quite large influences for taste perception. Just imagine a beer being served at two degrees higher temperature.
As stated, our question was “When it comes to determining the final temperature and dilution of a shaken drink, does the type of ice in the shaker or the shaking style really matter?” and our answer, and initial hypothesis was “No.”
Theoretically, our assumption was that the only way a drink gets cold in a bar is by ice melting. In a bar situation this is undoubtably true. Bar ice is at 0 (see below). Furthermore, the dilution and the chilling are linked because ice melting has a direct influence on drink dilution. What we didn’t know was how much the surface area to volume ratio of the ice mattered and how much the speed and style of agitation mattered.
The dilution measurements we made were direct. We measured the alcohol by volume of the drink in the glass using a refractomenter. The loss of liquid is not relevant to that reading. The use of colored ice wouldn’t help because we don’t know at what point in the shaking procedure the liquid is lost. Remember we are measuring an actual bartender doing an actual shake, not a theoretical one. If bartenders lose liquid, they lose liquid.
I don’t see how “everything is invalidated” because some liquid is lost in the shaking procedure. The temperature is the temperature and the abv is the abv.
I don’t understand the comment/question “where is the sink.” Nothing was dumped out. The one real problem with the whole thing is that “amount of ice melted” is intial ice weight (a known measured value)-finished ice weight (a known measured value) but since we don’t know at what point the liquid error occured, we don’t know if it happened pre or post dilution (ice melting) this could account for some of the movement between the melted amount and abv amount.
The whole thing about the ice being below zero comes from a misunderstanding of how commercial ice cube machines work vs home freezers. The ice in them never gets very far below zero to begin with (a couple of degrees), it is then stored in insulated but unrefrigerated large containers for hours and hours, and is then handled wet. Ie. at 0. These containers always have drains in them because the ice is always shedding water because it is always melting because it is at 0. This is the type of ice we used. I put in in a 0 degree cooling chamber just so it wouldn’t melt atrociously during the experiments, not really to warm it up. I would be more than happy to embed a thermocouple in a cube, but it was at 0.
The fact that ice at 0 can get an alcoholic drink colder than 0 is basic thermodynamics. It is the same principle as using salt and ice to make ice cream.
In a glass the situation is different from a shaker because there is no agitation. It is easier for temperature gradients to build up. If you take two alcoholic drinks below 0, sit them on a bench, put a bunch of small ice in one and once big ice cube of equal weight in the other, the small ice drink will stay cooler but become diluted quicker because there is a large surface area for cooling even without agitation, whereas the large ice cube has a smaller surface area and there isn’t enough agitation to have it reach its potential cooling power based on the volume of ice it contains. It is even more complicated than that because gradients of concentration (dilution) happen at the same time. You will have a water-rich area next to the cube.
I agree we should do more shaking. The graph you are referring to only has 16 shakes in it. I would like 5 times that number, but the temperature graphs we did have far more data embedded in them because we have constant temperature vs time measurements for dozens of shakes. We have no way as yet to measure abv as we are shaking.
I think your final note is valid. In fact it is the subject of not the next cocktail post, but the one after that. A degree of temperature isn’t so important in the range of -5 to -7 c, but the abv can be. The difference between 21 and 20 abv isn’t that great, but the difference between 20 and 19 is. Right now our evidence on this is somewhat anecdotal, but we are working on it.
Response to the hypothesis and the conclusion ‘no effect':
If I look at the graph of Alex Day I see reasonable differences. It takes about 50% more time to cool to a certain temperature when using kold draft.
Either this is seen as insignificant or this negates the initial hypothesis which states no differences are to be found. If one starts with a hypothesis as in no difference is to be found then it would be nice to state as well what sort of difference would be seen as a falsification. I can imagine very well that 50% difference could be seen as a falsification. If one would ascribe this 50% difference in the graphs of Alex Day to variations than one should not conclude that there is no effect but instead that the effect is not significant.
Reading further on the blog I saw that you believe that zero degrees things can cool other things below zero by themselves.
I have another doubt about your ice being exactly zero. If it would be at zero, why then would using more ice result in less dilution, as stated in your assumptions. If your ice would be really at zero degrees then it wouldn’t matter to use less or more ice as all of the cooling effect would be due to dilution when the ice would be really at zero degrees Celsius.
I don’t just believe it. It is true. Don’t believe me, believe the Gibbs free energy equation. Putting pure ice at 0C into an ice-alcohol solution at 0C will cause the whole mass to cool below 0C and cause the ice to melt.
The experiment is simple to do. Throw a bunch of ice into water. Make sure you have enough to get the water down to 0C. Leave it on the counter and wait till half of it melts. At this point i’m sure we’d all agree the remaining ice is at 0. Pull out the ice, shake off the water and put, say 200 grams of it in with 100 grams of vodka at room temp, and the whole thing will get well below 0.
By the way, even if my ice was 5 degrees below 0, there wouldn’t be enough excess negative temperature to drop my drink temp as much as we measured. Even though the heat capacity of pure ethanol is only close to half that of water, the specific heat capacity of ice is also only roughly half that of water, and the drink is at least 60 percent water to begin with… at 25 c.
Another interesting fact is that the thermal conductivity of ice is much much higher than water, so it equilibrates rather quickly and is an excellent chilling medium, even though it can’t equilibrate by convection or mixing like water can.
The more ice less dilution problem is interesting but has been noted by many many people. This is why any good bartender uses lots of ice. I think the reason is you need a certain minimum amount of ice in the drink to achieve anything close to an optimal chilling rate. Although I don’t think anyone has worked out that part of the problem fully. One thing many people have also noted is that, at a certain point, the benefit of adding more ice plateaus out. All of our experiments were done in that plateau. Eben Klemm has some data for establishing the plateau but I don’t have acess to it. Eben?
Fact is: two bodies are in (local) thermal equilibrium when they have the same temperature. No exchange of heat will take place.
The free energy potential assumes constant temperature which is not the case in this situation. Or… would you expect that 0 degrees ice cubes immersed in a -7 degrees liquid stay at 0 degrees?
The quick drop in temperature relatively far below zero makes me believe that somehow a lot of ice was used or very cold ice was used. This large drop in temperature within 5/10 seconds is amazing. Could possibly the thermocouple wire have given some errors?
The plateau for the decrease in dilution when adding more ice may be due to the distribution of the fluid on the ice having a certain limit. At some point adding more ice does not increase or increase much less the amount of fresh cold ice being in contact with the liquid as the liquid itself has a surface as well which is bounded. At this point the amount of dilution is determined by the time scale of the contact between the liquid and the ice. No matter how much ice you’ll add, the ice will still melt because the liquid will reside on an ice cube for some time, being able to increase the cubes temperature and melt it. If one would be able to spray the cocktail/liquid (increase it’s surface) and distribute it evenly over all of the ice than maybe no dilution would occur.
Do the experiment. Ice at 0C and water at 0C ARE at equilibrium, so nothing happens. Water + ethanol at 0C and ice at 0C ARE NOT in equilibrium. Just because they are at the same temperature doesn’t mean they are at equilibrium. The ice will melt. In order to melt, ice MUST absorb heat. The only place the heat comes from in a closed system is the system, so THE WHOLE SYSTEM COOLS DOWN. This is because it is entropically favored for ice at 0C to melt into water+ethanol. Seriously, do the experiment. Or do a google search on ice salt water entropy ice cream. This is a well known phenomenon.
Just to clarify a bit:
The freezing point of a pure compound is defined as the temperature at which a solid form of the compound is in equilibrium with a liquid form of the compound. Adding energy to the system will decrease the amount of solid as it melts without increasing the temperature of the system. Removing energy will increase the amount of solid without decreasing the temperature. Consequently, adding ice at 0C to water at 0C will not change the temperature of either. Any energy absorbed by ice that melts will be replaced by water freezing, and the energy balance of the system will be unchanged.
However, ice in vodka is not a pure compound. The ice has a freezing point of 0C, but the freezing point of vodka is considerably less. Consequently, energy absorbed by the ice melting will be replaced by the temperature of the liquid dropping rather than by new ice forming as it would if it was at the freezing point. The melting of the ice is driven by entropy so it will continue to melt (diluting the vodka and lowering the temperature of the entire system) until a new equilibrium is established. This equilibrium will be established at the freezing point of the ethanol-water mixture surrounding the ice, slightly higher than the freezing point of the vodka because the resulting solution, now that some ice has melted, is somewhat more dilute. This will still be higher than the freezing point of pure ethanol, and the ice formed will still be pure water (barring inclusions and other physical processes).
The hand-waving argument is that the entropy of the water molecules bound up in the ice is the same regardless of whether the ice is floating in vodka or in water, but the entropy of the water molecules in the vodka is less than it is in pure water. (Etropy is a measure of the amount of order, and there is more order in a system where water molecules are surrounded only by other water molecules than there is when some of them may have ethanol molecules nearby). For water in the vodka to associate into crystal structures, it must move around those pesky ethanol molecules until it is surrounded by all water molecules, which takes energy and means that it won’t happen until the temperature gets lower.
There is a full phase diagram in this paper:
http://www.nedo.go.jp/english/publications/reports/reitou/ps/p05.pdf
Actually, I see this as the strongest argument for using ice that is below the freezing point of the drink you are trying to make to cool your drinks. That would give you the most consistent dilution. The amount of ice melted would be a direct measure of the original temperature of the drink and its volume – any additional ice would remain in solid form, and as long as the volume of ice was at least large enough to reach that equilibrium, no additional ice would be melted. Using 0C ice, you have to consider not only the reduction in temperature of the drink to its freezing point, but also the reduction in temperature of the ice to the freezing point of the drink. Any additional ice added would require additional melting and therefore dilution to bring the total mass of ice into thermal equilibrium with the solution.
The entropy argument isn’t hand waving. Entropy is the true driving force of the phenomenon. The melting point of a pure substance is based on the Gibbs free energy equation. At the melting point, the amount of heat required to melt or freeze the substance (the Delta H) is offset by the change in entropy of the system (T Delta S). The ice in a cocktail is still pure ice –not an ice composed of ethanol and water (as the phase diagram of the paper you reference shows). The Enthalpy required to add a molecule of water or remove a molecule of water from the ice doesn’t change when you add it to vodka (the heat of fusion is the same). What changes is the entropy side of the equation (T delta S). Because the entropy gain of melting is greater in a water-ethanol system than a ater system at the same temperature, the ice melts and the temperature of the system drops. The drink will continue chilling and diluting till the entropy and enthalpy balance again.
Aha I see. There is a local thermal equilibrium between ice and liquid but the entire system is not in thermodynamic equilibrium. The liquid will cause the ice at the surface to drop in freezing point and melt without transfer of heat between the liquid and the ice. The melting will disturb the local equilibrium and allow heat to flow from the ice to the liquid.
The ice dropping in temperature is also a reason why there is a plateau in the effect of adding more ice reducing dilution. At some point the drink will be cooled below the temperature of the initial ice temperature. At this point having added more ice will be detrimental as more ice needs to be cooled which results in more dilution.
Any chance you’d want some blog coverage of the shake-off at Audrey’s?
Thanks for the link! What kind of coverage? BTW, we intend to do more as a group.
Another note:
The amount of added heat due to shaking is determined by the power input (which entirely dissipates and turns into heat) of the one who shakes the cocktail. The added cubes could make a difference if it would allow or influence the bartender to shake with more power but if he doesn’t (e.g. shaking till tired will be much the same in both occasions) than it won’t matter much. So, effect of type of ice on amount of generated heat… it depends on how the bartender shakes the mixer. One thing is for sure. If you shake water it’s temperature will rise. If you shake it harder it’s temperature will rise more (but the effect is not large, few people will shake much harder because there is different ice in the shaker).
Here is a link that should prove a little helpful:
http://www.engineeringtoolbox.com/ethanol-water-d_989.html
It describes the freezing points of ethanol/water solutions. For the sake of argument, let’s assume that our cocktail will dilute to about 20% ABV. The coldest we could theoretically make it is -9 degrees Celsius. (Without us even looking for that effect, you can see we came pretty close to that point-I suspect every bartender does without trying too hard.) This is part of what causes the plateau. The drink is cold, but the only way it can get colder is to freeze, and the action of shaking cannot put back in the amount of energy needed for it to make a phase change. So it’s stuck.
The other factor in the plateau is caused by the increased dilution of the water. Even though the higher the water concentration, the more in range a potential freezing point seems to be; but at the same time the specific heat of the solution becomes higher, and it becomes more difficult to move the solution’s temperature either way.
This is one of the reasons that life as we know is possible so we really ought to be rather grateful.
Martijn:
Your comment about a 50% difference in Alex’s shake temperatures between ice sizes is rather like watching the 1st 10 meters of last weeks 100 M World Championships and saying Usain Bolt tied.
In a real world bar setting (and even in our surreal lab setting), every serious bartender works into their motions slightly differently. We started our timecourses at the moment we hit the shaker cans together. Please permit us our time to raise the shaker to our chests, wink at the guest, and commence our shake. We all ended up in the same place.
And as for your post beginning “The ice dropping in temperature”…no. Unless I am totally misreading what you are trying to say, the ice does not cool down the drink and then get recooled by the drink. That would be perpetual motion.
Something has been puzzling me about your combined chart showing ice melted, %ABV and temperature. . . Looking at the data points, we see that amount of standard ice melted increases sharply between 8 and 9.5 seconds. In addition, see that the %ABV for the standard ice sample also increases sharply between 8 and 9.5 seconds. How is this possible? More water melted in equals lower %ABV, not higher. Indeed, I don’t quite understand any of the reverse turns in dilution and ice melted. Is it possible that the sample size was too small for a good average and these values are being unduly influenced by outliers? With a sufficiently large sample size, I would expect to see more or less smooth curves showing a steep incline or decline at the beginning and then tapering out over time to some asymptote.
I think the deviation in ice melted comes from the shaking loss we experienced. The ice melted number is a calculated number equal to the weight of the inputs minus the weight of the drinks. That number includes the shake loss. The abv follows the expected trend because the loss in liquid was probably early in the shake rather than later. I’d like to run the test again in a completely sealed system instead of actual cocktail shakers but haven’t had the time. ABV, temperature, and drink weight are not calculated, they are measured.
One thought I just had. You may want to shake using gloves to insulate the shaker from body heat. Of course, if you are going for typical results, then you’d want to forego the gloves.
Blair
Hi Dave,
the experiment you guys made is absolutely agreeable with what i have experienced for the last 11 years as a bartender (although big and solid ice cubes tend to make the drink nice and frothy if it contains pulp or juices or other proteins). the thing that really puzzles me though is your ” using different shaker configurations” graph and the conclusion that metal shaker achieved lower final temperature than glass. in my experience at work and here i must say i never approached this scientifically, when shaking in the glass-steel boston shaker frost always forms on the outside of the thin, but whenever i shake that same cocktail between two thins i can never get them to form frost on the outside.
however your post is as always brilliant and educational. Thank you
Interesting.
Dave… do you know how to make the ice cubes that people use at home clear instead of cloudy? I heard that boiling purified water, letting it cool, boiling it once more and letting it cool again does the job. This technique supposedly removes impurities. However, after trying this technique, I can say that it doesn’t make the ice cube fully translucent. It only helps slightly. Your thoughts, methods? Are clear ice cubes made from distilled water perhaps?
Ice cubes have three separate problems:
1. Water contains impurities that cause disruptions in the crystal structure and cause cloudiness. Distillation will help with this.
2. Water contains air which provide places for the crystal structure to be disrupted. Boiling and vacuuming helps this, but simply pouring water from one container to another can cause problems.
3. Your freezer lets the top of the ice freeze first. As the center of the ice cube freezes it expands breaking through the crystals above it and ruining everything. This is why the pros (like clinebell) freeze from the bottom and constantly circulate the water on top. This 1. stops the top from freezing and 2. keeps the impurities from settling on the bottom and providing a place for cloudiness to happen.
I once put a circulator in a chest freezer and froze a 30 gallon block of ice. It looked great except the ice froze from the sides. Most of it was super clear except for a concentrated dirt tornado in the very center. Antigriddles don’t have enough power to freeze a really big cube. Iv’e heard antigriddle plus chest freezer plus circulator works.
At home try using boiled distilled water in super clean large containers. Agitiate the top every once and a while to prevent freezing or put an aquarium pump at the top. Be prepared to lose the top portion of the ice.
Hope this helps.
Best,
Dave