posted by Dave Arnold
One difference between simple agar clarification and gelatin clarification is that gelatin clarification has the ability to clarify and concentrate at the same time while simple agar clarification does not.

For those who don’t know agar clarification see here. For those who don’t know how to clarify liquids with gelatin, here it is: hydrate approximately5 grams of gelatin into 1 kilo of liquid, freeze the liquid solid, and slowly thaw it over cheesecloth in a refrigerator.Â
One of the interesting properties of gelatin clarification is that the first liquid that drips out of the frozen block is extremely rich in sugar, acid, color, etc. It is concentrated. As the block continues to thaw, the liquid becomes more and more watery. This process allows for an easy way to concentrate flavor without using heat, and to adjust the strength of the clarified product (at the expense of yield) by terminating the clarification process whenever desired.Â
Simple agar clarification doesn’t work this way. The new clarification technique is simple forced syneresis (the leaking of fluid from a gel), and doesn’t preferentially drip sugar, acid, and flavor the way an ice cube does. The agar does hold on to some water (that’s what a hydrocolloid does), so the clarified product is more concentrated than the original; but the product’s concentration doesn’t change over the course of clarification the way it does with gelatin.Â
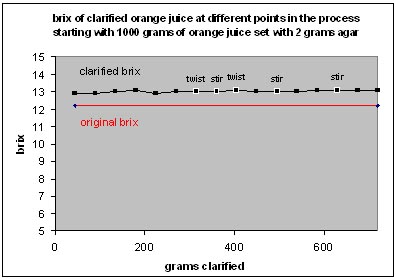
To prove it we hydrated 2 grams of agar in a kilo of orange juice, set it, broke it up with a whisk, and drained the clarified juice in 45 gram increments in mise en place cups and measured the Brix of each sample with a refractometer. We also tasted the samples to see if there were any appreciable difference.
Results: The original Brix of the juice was 12.2. Brix of the samples fluctuated between 12.9 and 13.1 (not much of a difference). We noted the moments we twisted extra hard or re-stirred the agar, but that didn’t make a difference either. The samples all tasted about the same. Juice from the end of the run has a slight haze (from pressing really hard on the agar and extruding some through the cheesecloth). Finally, they all tasted about the same. Here is a graph:
harvesting gelatin clarifications prematurely is dangerous business, fraught with an angry chef and inconsistent consommes…proceed at your own peril
Ha Ha. I had a discussion with Wylie on this point. He has had a lot of problems with people pulling gel clarifications early. He thinks the flavors are unbalanced, inconsistent, and unpredictable with partially thawed clarifications. He likens it to using a partially brewed pot of coffee. Point taken (although I like ristrettos). I, however, have gotten good results with products like grapefruit juice.
Hi,
this clarification stuff sounds extremely interesting to me (a research chemist and hobby chef).
Your comments on the concentration gradient of your flavors in the gelatin method got me thinking, and I believe that I have a good explanation for this.
What is more this could lead to some improved technique if you are willing to try!
For simplification let’s consider your stock to be simple salt water.
What happens in the freezing process?
As soon as the temp is low enough (a few degrees below 0°C due to the freezing point suppression of the salt) ice crystals will form (pure ice). The broth around them crystals will increase in salt concentration. Eventually the crystals will start incorporating more and more salt, proteins, flavor you name it, as the block freezes completely. At this point in time the freezing point of the last, dirtiest ice crystals is significantly lower than that of the first pure ones.
Note that the gelatin is not needed for my reasoning at all…
Now in the thawing process the stuff with the lowest freezing point melts first.
Therefore you first release lots of flavor.
(This can be observed with any water based “ice creamâ€: in Germany we have a brand of basically frozen orange juice on a wooden stick. If you (instead of biting chunks of) start sucking the juices out of the crystal conglomerate, the fist juice is extremely intense in flavor, and after a while you are left with some boring white and essentially tasteless block of watery crystals.)
Finally my proposed experiment / potential improvement for your procedures:
You do not need the thawing process for the concentrating up. This already happens in the freezing cycle!
Therefore you could let your stock freeze half way (best with occasional stirring to get large crystals but keep them from locking together too quickly.)
Now the flavor is enriched in the stock, the crystals should be depleted of taste.
Pass this quickly thru a cheesecloth (don’t let them crystals thaw).
Voila, rapid concentration of flavors.
Second step could be clarification by your brilliant method.
You still save a lot of time for the thawing!
Good luck and let me know if you find the time to try this.
Schinderhannes
I love the the things you do, but with all of these techniques, I challenge you to do something so savvy which makes dining more accessible to those who cant afford $300 a couple for a dinner.
These techniques if mass produced should be a gateway for elegant cuisine for the masses.
Following your post of the nut puree centrifuge experiment… one proposed explanation of why adding simple syrup to a nut puree pre-centrifuge would be that sugar has a tendency to bind to things really well and as sugar is heavier than oil (per same level of volume) than it only makes sense that the oil would be clearer because of the fine particulate removal still floating around being bound up and pulled down by the sugar, just a thought, hope it helps.
Dave,
I apologize in advance if my question seems stupid or philistine, but why (other than “it looks cool” would you want to clarify a liquid?
I can think of a few, perhaps you don’t want any gelatin in your stock, or maybe for some reason you don’t want any oil (e.g. hot peppers). Does it have other effects, and are they readily predictable?
Hi Jeremiah,
Well, clarified stuff does look fantastic, and sometimes that is reason enough –fruit soups, etc–; but there are other reasons. Clarified products tend to have a different mouthfeel from unclarified products (cause the stuff in them that made them cloudy has been removed). Usually, the mouthfeel is lighter, smoother. Also, the clarified products usually taste “cleaner.” For instance, clarified grapefruit juice tastes clean and bright, and has lost some of the bitter components of the juice without losing characteristic grapefruit flavor. One last point: carbonation works much better on clarified products (we cabonate a lot). Unclarified products tend to foam a lot because all the turbid stuff provides nucleation sites for bubbles.
Dave,
Thank you very much for:
1) You lucid answer
2) Entertaining my n00b questions
3) Your fantastic blog
Agar and a scale going on the shopping list…
Curiosity…
I sort of fell in love with your website, and all the ideas it ventilates.
Unfortunately I am currently unable to do kitchen chemistry, simply too busy doin the conventional stuff.
Do you think you will find time to try the semi freezing / concentrating up (either pre or post Agar clarification) of some juice or stock.
I strongly feel this must be the way to the most concentrated clarified lime juice ever!
It would be possible to do a fast freeze (LN) of the agar block and then a force-thaw at 30C. That should concentrate it. Another option would be to do simple agar plus rotovapping. Save volatiles, throw away water, add back volatiles. One problem I see is that part of lime juice’s instability is due to concentration. It has been years since I read the papers, but I think lime juice isn’t protected by normal freezing or by vacuum packaging. I know from experience that the acid residue in the distillation flask doesn’t taste good. I always attributed that to just being warm (40C), but perhaps it was being degraded due to concentration? I dunno. What’s also intersting is that lime volatiles degrade when left by themselves but are more stable in a mixed drink. I guess I need to do more research.
Rotavapping won´t due it, too delicate, when you ned to cath volaites and residue and cut out water in the middle, I bet you will be loosing plenty of taste on both ends, plus you need to heat.
Also why force freeze and force thaw?
Semi freeze slowly to generate large crystals of water in the freezing, drain juce of them crystals (of nearly pure water), done!
Hannes
I used to do lime juice rotovapping all the time. I guess there is no way to measure how much flavor I lost. I used to throw away the stuff in the distillation flask and mix the volatiles with a 2:1:0.1 Citric,Malic,and Succinic acid mix. We blind tasted against lime juice and used to win! I did use twice as much volatiles. So to make 500 ml of clear rotovapped lime I would use 1000 ml of fresh lime.
Another idea for some lab equipment that might be useful in the kitchen:
If you take a cross flow ultra filtration membrane and pump some clarified stock over it, you will be able to do two things with this rig:
as ultra filtration membranes allow small molecules namely water, but also e.g. alcohol and simple ions (Na+ Cl-) to pass thru, whilst retainig proteins (the taste of you broth), you can desalt and concentrate a stock by dong so. (Extremly mild process, used for peptidic drugs).
Might be fun to produce the mother of all consommes this way!
Later on you can salt it with any freaking salt you want, to the precize perfed taste.
good luck…